We know Mid-Year Exams are just around the corner for most ‘O’ Level Chemistry Secondary 3 and 4 students.
Some of you may be feeling anxiety, or some might feel that while you study really well in class, you just aren’t getting the resources you need on the internet.
Read also: O Level Physics: Ultimate Study Resource – Tips, Cheatsheet, and Summary Notes
Tutopiya’s Team of Experts has already broken down the guide to Chemistry for ‘A’ Levels and even helped your child prepare for the upcoming SA1 Primary School Examinations.
But we still have the GCE O Levels, which are crucial for your child to secure a top spot in a Junior College or Polytechnic of their choosing.
That’s why we’re breaking down systematically the top topics you’ll be tested for the O Level Chemistry Mid-Year Exam, especially if you are a secondary 3 or IP student.
Download the entire summary notes here!
Mid Year Examination Revision for O Level Chemistry
Atomic Structure

(Source)
Isoelectronic: Isoelectronicity happens when two or more molecules have the same structure and the same electron configurations. However, they differ by what specific elements are at certain locations in the structure.
Isotopes: Isotopes are two or more types of atoms that have the same atomic number and position in the periodic table. They differ in nucleon numbers due to different numbers of neutrons in their nuclei.
Finding the relative atomic mass of isotopes
- Add an abundance of the first isotope multiplied by its atomic mass to an abundance of the second isotope multiplied by its atomic mass and so on and so forth and then divide it by the number of isotopes.
- The formula is as follows:
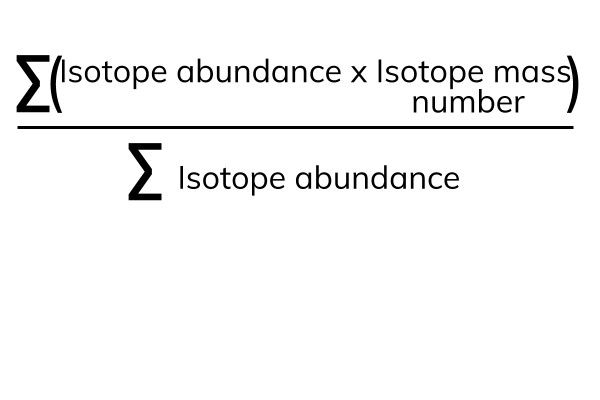
(Source)
Electronic structure
– s,p,d,f,g orbital notation
Aufbau Principle
– Electrons fill up orbitals of lowest energy first before proceeding to higher energy levels
Pauli exclusion principle
An orbital can only hold a max of 2 electrons of opposite spins
Hund’s rule of multiplicity
Electrons occupy different orbitals each first before pairing up in each orbital
| 1s,2s,2p,3s,3p,3s,4s,3d…… |
Overlap in energies of certain layers: 4s and 3d, 3d is higher
In the case of ions drawing electrons will draw from 4s first before 3d even if it’s a higher orbital
s = 2, p =6, d=10, f= 14
Can write using noble gas core
1s2,2s2,2p6,3s2 as [Ne] 3s2
Separation Techniques
Filtration
– A separate mixture of insoluble solid and liquid
– Mixture poured through filter paper
Sublimation
– Change of a state of a substance from solid to gaseous without going through the liquid state on heating
– Mixture is heated gently, sublimate condenses on cooler sides of the funnel
Distillation
Fractional distillation: separate a mixture of miscible liquids using a fractionating column
Separates liquids in order of boiling points
– Liquid with lowest boiling point distilled first
– Liquid with highest boiling point distilled last
Chromatography
Separate and identify both colored and colorless mixtures
The mixture must be dissolved in the same solvent
Components travel at different rates over the paper, depends on solubility in the solvent. The more soluble it is, the faster it moves.
Rf value = Dist. Moved by substance/ Dist. Moved by solvent.
Chemical Bonding
Dot and cross diagrams
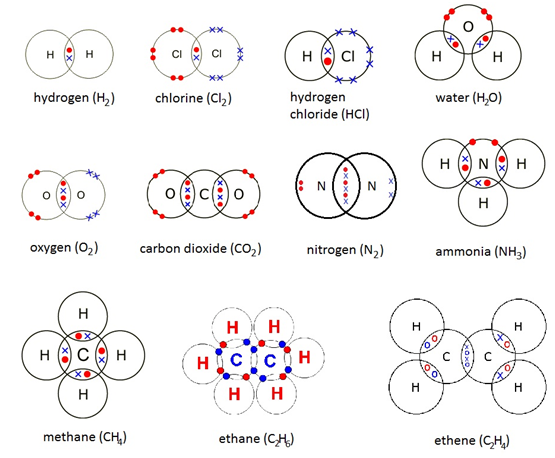
(Source)
Ionic Bonding
The electrostatic force of attraction between two oppositely charged ions.
They are usually formed between non-metals and metals.
They have high melting and boiling points.
– Due to the strong electrostatic forces of attraction between oppositely charged ions in the giant ionic crystalline lattice structure, a large amount of heat energy is needed to break these bonds
Ionic substances conduct electricity in an aqueous and molten state but not in solid
– In a solid-state, ions are held together by a strong force of electrostatic attraction between oppositely charged ions. They can only vibrate around their own position.
– In the liquid state, the strong forces of electrostatic attraction between oppositely charged ions are broken. Ions are free to move around and Electricity can be conducted by these mobile ions. They are also soluble in water.
– Water molecules can bond with both positive and negative ions, breaking up the lattice structure
Metallic Structures
– Metallic structures consist of positive ions in a sea of delocalized valence electrons
– Metallic ions and a sea of delocalized valence electrons have a strong force of electrostatic attraction between them
– Can conduct electricity: Sea of delocalized valence electrons can move around freely and help to conduct electricity
Covalent Bonding

(Source)
Two main types of Structure: Giant and Simple Molecular structures
Between two non-metals
Giant Molecular Structure
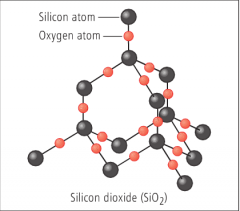
(Source)
– Covalently bonded: Millions of atoms joined by strong covalent bonds through the structure
Classic examples: Diamond, SiO2
– High melting and boiling points\Hardness
The whole structure is held together by a network of strong covalent bonds between atoms.
A large amount of energy is needed to break these bonds.
Exceptions to normal GMS: Graphite
Layered structure, between layers of strong covalent bonds, there is weak Van Der Waal’s force. Van Der Waal’s force can be overcome easily and thus graphite is soft and easily broken.
Can conduct electricity
There is a non-bonded valence electron on each carbon atom as only three covalent bonds are formed, thus these electrons can help to conduct electricity
Simple Molecular Structure
(Source)
Strong covalent bonds between atoms within a molecule but weak intermolecular forces(Van Der Waal’s force or H-bonding) between molecules.
Low Melting and Boiling point
Due to weak intermolecular forces of attraction between molecules
Little heat energy is needed to overcome these intermolecular forces of attraction.
Non-conductor of electricity
Neutral molecules that do not contain mobile ions or delocalized electrons.
Exceptions of cases: Hydrogen Bonding
– N, O, F
Hydrogen with N, O, or F usually results in a covalently bonded atom with hydrogen bonding between molecules as well.
While they have Van Der Waal’s forces similar to normal Simple Molecular structure, they are also held together by hydrogen bonding and thus need more energy to overcome these forces.
Ionic Equations
Ionic equations are chemical equations that have the non-participating ions removed.
Key points
– Do not remove solid/liquid/gas reactants/products
– Remove aqueous reactants/products or parts of them that do not end up as a solid, liquid, or gas in the final equation.
– Add charges to aqueous reactants/products that have had their counterparts removed.
HCl (aq) +NaOH (aq) -> NaCl (aq) + H2O (l)
– HCl and NaOH are aqueous. However, H and O become H20, a product that is a liquid, thus they are not removed but Cl and Na are.
Ionic Equation : H+(aq) + OH– (aq) -> H20 (l)
Acids, Bases, and Salts
Acids
– A substance that produces H+ ions as the sole positive ion in water.
– Weak acids do not disassociate fully, strong acids disassociate fully
– Turns blue litmus paper red
– <7 on the pH scale
Reactions with other substances
– React with metals
Products are hydrogen gas and salt
– React with carbonates
Products are carbon dioxide, salt, and water
– React with metal hydroxides/oxides (Neutralisation)
Products are salt and water
Bases
A substance that produces OH- ions as a sole negative ion in water
Alkali = soluble base
Turns red litmus paper blue
>7 on the pH scale
Reactions with other substances
React with acids (Neutralisation)
Products are salt and water
React with ammonium salts
Products are salt, water, and ammonia gas
Alkali reacts with a metal salt
Products are metal hydroxide and salt
Oxides
Metallic oxides
Basic Oxides
Reacts with acid to produce salt and water
CaO, MgO
Amphoteric Oxides
Reacts with both acids and bases
ZnO, Al2O3, PbO
Non-metallic oxides
Acidic Oxides
Reacts with alkalis to produce salt and water
NO2, CO2, SO2
Neutral Oxides
Does not react with either alkali or water
CO, NO
Solubility Rules
| Nitrates | All are soluble |
| Sulfates | All soluble except Ba, Ca, Pb |
| Chlorides | All soluble except Ag, Pb |
| Carbonates | All insoluble except Group 1 Metal and ammonium carbonates |
| Hydroxides and Oxides | All insoluble except Group 1 Metal and some Group 2 Metals |
| Reactivity of metals | Most reactive: K, Na, Ca, Mg ,Al |
| Most non-reactive: Gold, Platinum, Silver, Copper | Preparation of Salts |
Qualitative Analysis
| Test for cations | |
| NaOH | White precipitate produced
Insoluble in excess Ca Soluble in excess Al, Pb, Zn Green precipitate produced Fe 2+ (insoluble in excess) Reddish-brown precipitate produced Fe 3+ (insoluble in excess) Blue precipitate produced Cu 2+ (insoluble in excess) |
| NH3 | No precipitate
Ca White precipitate produced Soluble in excess Zn Insoluble in excess Al, Pb Fe 2+, Fe 3+ and Cu 2+ are similar to reactions in NaOH |
| Note: To identify between Al and Pb, react both with chloride ions. Al with chloride ion produces white ppt while Pb with chloride ion produces a colorless solution. | |
| Test for anions | |
| Carbonate | Add dilute acid: Carbon dioxide, water, and salt is produced |
| Sulfate | Acidify with dilute nitric acid, add aqueous barium nitrate
White ppt formed |
| Chloride and Iodine | Acidify with dilute nitric acid, add aqueous silver nitrate/lead (II) nitrate)
Chloride: White ppt, Iodine: Yellow ppt |
| Nitrate | Add aqueous NaOH and aluminum foil/Devarda’s alloy and then warm gently
The effervescence of colorless and pungent gas turns moist red litmus paper blue. |
Test for gases
| Test for gases | |
| Hydrogen | A lighted splint (extinguished with pop sound) |
| Oxgen | A glowing split (rekindles the splint) |
| Carbon dioxide | Limewater (white precipitate is formed) |
| Sulfur dioxide | Add acidified aqueous potassium dichromate (VI)
The effervescence of colorless and pungent gas turn moist blue litmus paper red. Orange acidified potassium dichromate (VI) turns green |
| Chlorine | Observation: Effervescence of greenish-yellow and pungent gas which turns moist blue litmus paper red and then bleaches it. |
| Ammonia | Observation: Effervescence of colorless and pungent gas that turns moist red litmus paper blue |
Chemical Calculation

(Source)
1 mol = 6.02 x 10^23
No of moles = Mass(in g)/Molar mass
-
- Note: When facing diatomic molecules, need to multiply >.>
For finding Empirical Formula and Molecular Formula
Lithium forms a compound with a composition of 8.00% lithium, 36.8% sulfur, and 55.2% oxygen. (a) Find the empirical formula of this compound. (b) The relative molecular mass of the compound is 174. Find the molecular formula of the compound.
| Li | S | O | |
| Mass [in 100g] | 8 | 36.8 | 55.2 |
| Molar mass | 7 | 32 | 16 |
| Mols | 8/7 = 1.14 | 36.8/32 = 1.15 | 55.2/16 = 3.45 |
| Mole ratio | 1 | 1 | 3 |
Empirical formula = LiSO3
Molecular formula = n x empirical formula
n = 174 / [7 + 32+ 16×3]
n = 2
Therefore, molecular formula is (LiSO3) x 2 = Li2S2O6
Molar gas volume = 24 dm3
Molarity
Concentration in mol/dm3 x molar mass = concentration in mass/dm3
Chemical Periodicity
Metals reactivity increase down the group
Atomic size of metals increases, atoms can lose valence electron easily to form positive charge ions
Non-metals reactivity decreases down the group
Electronegativity increase àand up the group so F would be most electronegative
As Mr increases, Van Der Waal’s force gets stronger and stronger, thus covalent compounds further on the table would have higher boiling and melting points.
Group I – Alkali Metals
Properties
– Shiny, silvery, metallic solid (metal)
– Soft
– Melting point decreases down group
– Li, Na, and K have low density, can float in water.
Reactivity increases down table
Group VII – Halogens
Appearances
| Elements | Chemical Formula | Colour | State |
| Fluorine | F2 | Yellow | Gas |
| Chlorine | Cl2 | Greenish yellow | Gas |
| Bromine | Br2 | Reddish Brown | Liquid |
| Iodine | I2 | Black | Solid |
| Astatine | At2 | Black | Solid |
Properties
| Exists as diatomic molecules |
| Simple molecular structure with weak Van Der Waals’ force existing between molecules |
| Reactivity decreases down table |
| Displacement reactions
More reactive halogen displaces less reactive halogen from compound Cl2(g) + 2 NaBr (aq) -> 2 NaCl (aq) + Br2 (l) |
| Group VIII – Noble Gases
Unreactive monoatomic gases Very stable electronic structure All have low melting and boiling point- Van Der Waal’s force |
Gases can be used to act as inert atmosphere
| Argon and helium | During welding, if aluminum is welded in the air, hot metal will catch fire and burn in oxygen.
Argon and helium provide an unreactive atmosphere to prevent this. |
| Transition Metals | Strong hard metals with High Density
High Melting and Boiling point – Metallic bonds Variable oxidation state Forms colored ion: Iron (II) – Pale green, Iron (III) – Pale yellow, Copper (II) – Blue Use as catalysts Exceptions Scandium, Zinc(Most likely one to be tested), Silver Only one oxidation state: Zn2+ Form white compounds in solid states Colored compounds in aqueous states |
Atmosphere and Environment
Air: 79% Nitrogen, 20% Oxygen, 1% Noble gas, 0.03% Carbon Dioxide, 0.5% Water Vapour
Air pollution: Chemicals in air with high enough concentrations that it that harms living organisms
Pollutants : Harmful substances found in environment
Carbon monoxide
– Source: Forest fires, incomplete combustion of fuel in cars
– Reacts with haemoglobin, forming stable carboxyhaemoglobin which prevents blood from transporting oxygen
– Paralyzes brain activity, headaches, fatigue, impaired judgement
Colourless, tasteless, odourless
Sulfur dioxide
Source: Volcanic eruptions, burning of fossil fuels with sulfur as impurity
Poisonous choking gas: Irritates eyes, attacks lungs , causing breathing difficulties, leading to bronchitis
Forms acid rain
Sulfur dioxide dissolves in water, forming sulfurous acid, H2SO3
Sulfurous acid oxidizes to form sulphuric acid (sulphuric acid rain)
Corrodes metal and limestone structures
Poor health and stunted growth in fish
Absorption of needed nutrients by plants affected, replaced by toxic ions, killing plants/
Oxides of Nitrogen
Source: Car exhaust fumes (high temperature in the engine causes nitrogen and oxygen to react) and lightning (through heat released by lightning)
Nitrogen Dioxide: red-brown toxic gas, unpleasant pungent odour
Causes eye irritation, damage lung tissues, and blood vessels
Forms acid rain
4NO2 + 2H20 + 02à 4HNO3
Lead
Lead accumulates in the body, causing damage to the brain, liver, kidneys, central nervous system
Symptoms: Loss of appetite, vomiting, convulsions
Methane
Source: Bacterial decay of vegetation, fires, mining, decaying animal dung, rubbish in landfills
The colorless, odorless gas
Under strong sunlight, reacts with nitrogen dioxide forming photochemical smog
Greenhouse gas causes global warming
Unburnt hydrocarbons
Source: Incomplete combustion of petrol in car engines
The component in photochemical smog
Photochemical smog
A mixture of pollutants: dust, nitrogen oxides, ozone, unreacted hydrocarbons, peroxyacyl nitrates (PAN)
Brownish haze, painful eyes, reduced visibility
Causes headache, eye , nose, throat irritation, impaired lung function, coughing and wheezing.
Ozone
Combines with unburnt hydrocarbons to form PAN, which causes tearing of eyes
– Dangerous of asthmatic patients
– Damage rubber in car tires and fabrics
– Damage plants
Ozone layer depletion
The ozone layer acts as giant sunscreen to protect Earth’s surface from harmful UV radiation
CFCs: aerosol, refrigerators, cleaning solvents
CFC molecules rise into upper atmosphere, forming chlorine atoms, chlorine atoms react with ozone molecules
Ozone layer is destructed, UV rays are let through, causes skin cancer, eye cateracts , severely damages plant growth
Catalytic converter
Contain catalysts: platinum and rhodium
Carbon monoxide reacts with nitrogen oxide to form nitrogen and carbon dioxide
Unburnt hydrocarbons oxidized to carbon dioxide and water
Found in cars mostly to reduce pollution caused
Ways to minimize acid rain
Treat soil with calcium carbonate/calcium oxide to neutralize excess acidity
Flue gas desulfurization
– Sulfur dioxide is removed from flue/waste gases by reacting with an aqueous suspension of calcium carbonate, forming calcium sulfite
CaCO3 + SO2 àCaSO3 + CO2
Calcium sulfite oxidized to form calcium sulfate.
Download the entire summary notes here!
Download Here!
O Level Chemistry Tuition
Check out Tutopiya’s post on (Most Neglected) Math Tips In Math Exams | GCE O Level AMath and EMath.
Tutopiya is a Singapore tuition agency that offers 1-1 live online tutoring platform for students to learn comfortably anywhere anytime.
Tutopiya offers live online tutoring, allowing you to learn anytime and anywhere. Technology advancement has greatly improved our standard of living such that now, even without leaving the house, we can learn.
Good tuition comes with good tutors.
Tutopiya’s tutors are highly trained and are Graduates from the Best universities. Sign up for a FREE 1-1 trial class now!

About The Author
Nuha Ghouse
Nuha Gouse is the Co-founder of Tutopiya and is equipped with a first class honours Math degree from Imperial College, London. Her mission is to provide personalized individual lessons online where students from around the world can learn at their own pace and convenience.




